The Center for Biologics Evaluation and Research (CBER) serves a vital role in protecting and advancing public health by ensuring the safety, purity, potency, and effectiveness of biological products. As a part of the U.S. Food and Drug Administration (FDA), CBER regulates a wide range of biologics, including vaccines, blood and blood products, cellular and gene therapies, and tissue-based products. Its oversight helps ensure that these complex and often cutting-edge medical products meet rigorous standards before reaching the public.
The educational materials presented on this platform are derived directly from previous events hosted by the Center for Biologics Evaluation and Research (CBER), such as webinars and conferences. For ease of learning, this content has been organized and broken down into smaller sections, including presentations and speaker information. Please note that this website is a third-party resource and is not affiliated with the FDA or CBER.
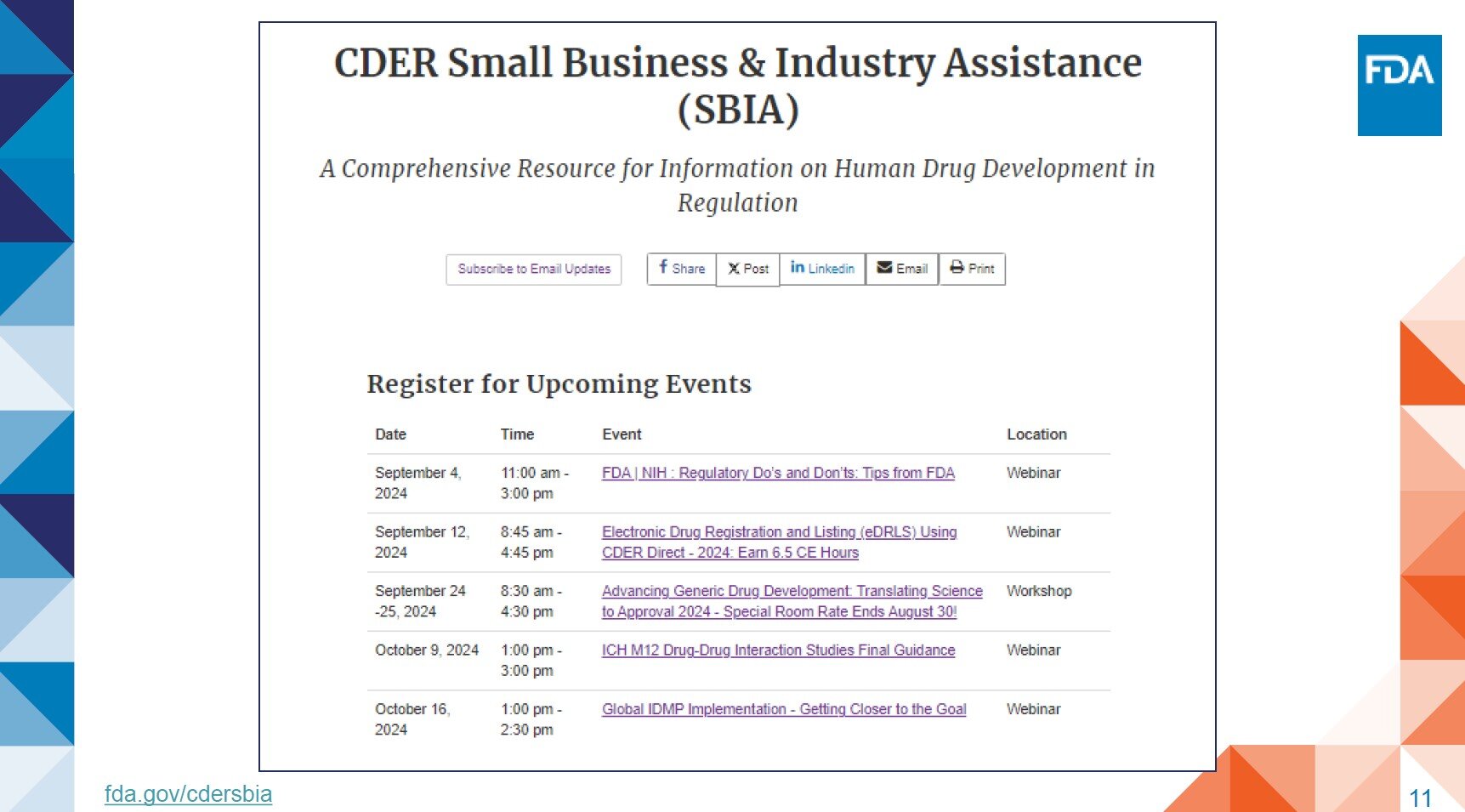
Additional Resources:
- CBER Workshops, Meetings & Conferences
- CBER Webinars and Outreach
- OTP Events, Meetings, and Workshops
- FDA Meetings, Conferences and Workshops
- FDA Employees CE Portal
- FDA Grand Rounds