The Center for Devices and Radiological Health (CDRH) promotes and protects public health by ensuring the safety, effectiveness, and quality of medical devices and radiation-emitting products. As part of the U.S. Food and Drug Administration (FDA), CDRH oversees a broad range of products—from simple items like tongue depressors to complex technologies like pacemakers, diagnostic imaging equipment, and software used in healthcare. CDRH also monitors products that emit radiation, such as X-ray machines and microwave ovens, to ensure they meet safety standards.
The educational materials presented on this platform are derived directly from previous events hosted by the Center for Devices and Radiological Health (CDRH), such as webinars and conferences. For ease of learning, this content has been organized and broken down into smaller sections, including presentations and speaker information. Please note that this website is a third-party resource and is not affiliated with the FDA or CDRH.
Additional Resources:
- CDRH Learn
- Device Advice: Comprehensive Regulatory Assistance
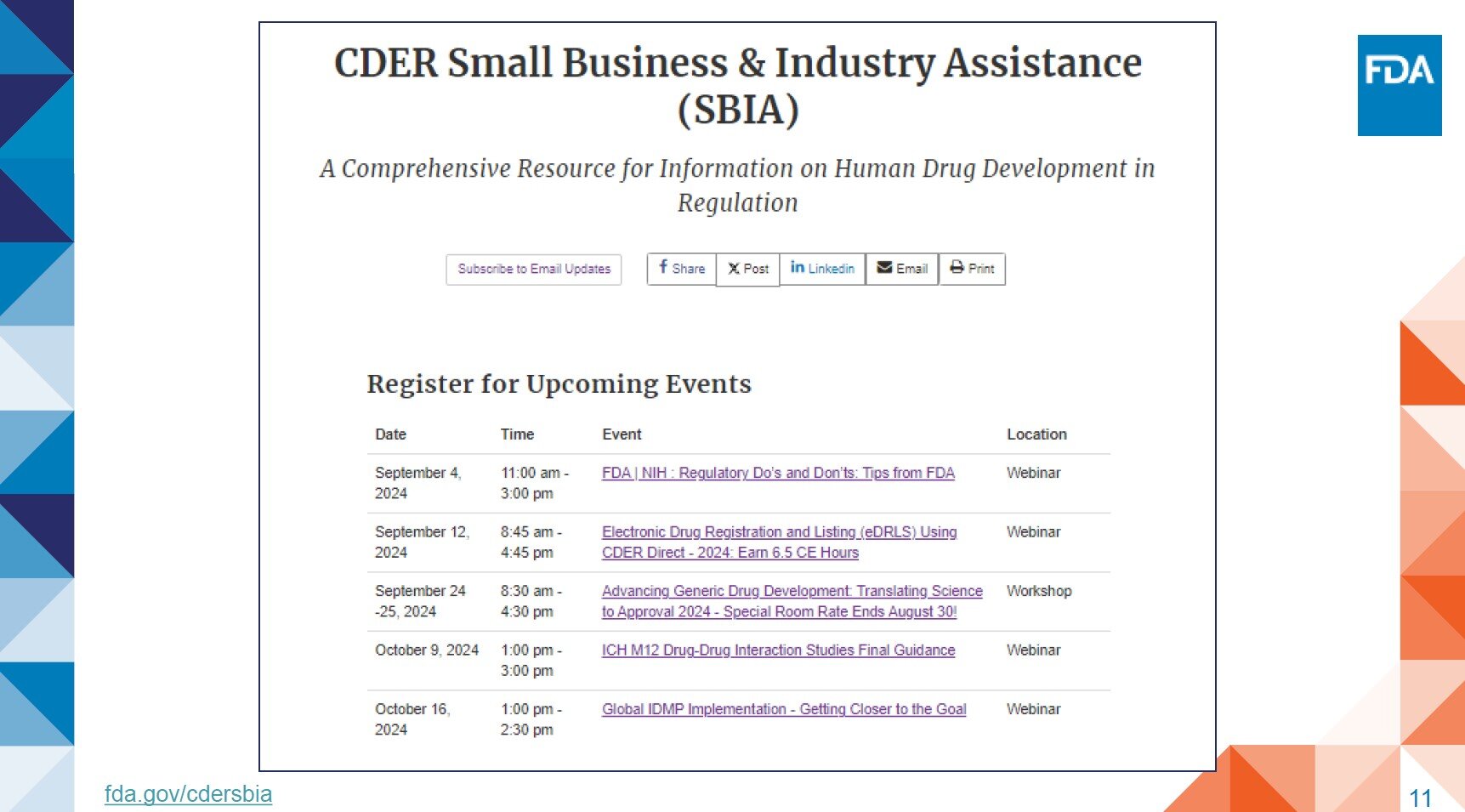
- CDRH Events
- CDRH Training Curriculum for Third-Party Reviewers
- FDA Meetings, Conferences and Workshops
- FDA Employees CE Portal
- FDA Grand Rounds